Thesis by Mohammed Bin Jassar, winner of the 2024 Yves Chauvin prize: « Mieux comprendre la formation et la croissance de la Solid Electrolyte Interphase dans les batteries Li-ion par une approche de modélisation moléculaire » (Gaining a better understanding of the formation and growth of the Solid Electrolyte Interphase in Li-ion batteries via a molecular modeling approach).
The gradual loss of autonomy of lithium-ion batteries, used in our cell phones and electric vehicles, is notably linked to the formation of a layer known as a Solid Electrolyte Interphase (SEI), which builds up between one of the electrodes and the battery electrolyte. The formation of this SEI layer (Figure 1) was studied theoretically in this thesis using molecular modeling to improve the understanding of its thermodynamic and kinetic aspects.
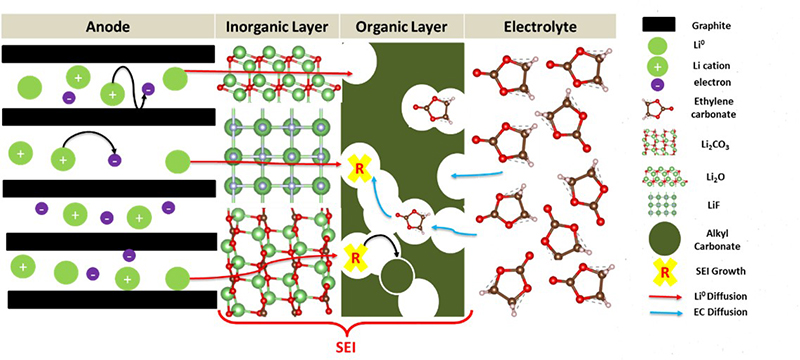
and the inorganic and organic layers forming the SEI layer and the electrolyte.
The PhD research initially focused on compiling a database of the principal degradation reactions involved by calculating the reaction and activation energies using DFT1. The results used in KMC2 simulations then revealed that salts such as Li2CO3 and Li2O, derived from electrolyte degradation, play a crucial role. Simulations are also used to predict the loss of battery capacity as a function of the initial composition of the SEI layer (Figure 2) and hint the importance of considering other salts such as LiF resulting from degradation [1].
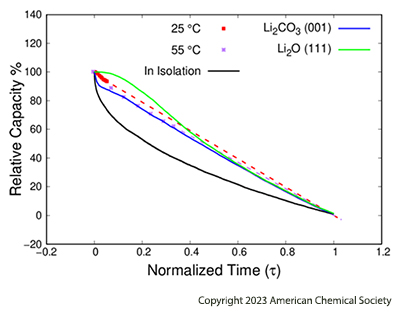
(Reprinted with permission from [1] - Copyright 2023 American Chemical Society)
The second aspect of the thesis focused on the evaluation of semi-empirical methods, which are less costly in terms of computing resources than pure DFT. Among these methods, GFN-xTB3 has proved to be particularly promising, as it leads to much shorter computation times, with only a slight loss in terms of result precision. In particular, this method can be used to model electrolyte degradation reactions, the insertion of lithium ions between the graphene layers of the anode, the growth of Li salt nanoparticles (Li2CO3, LiF, Li2O) and the interaction between various organic electrolyte components and these inorganic nanoparticles [4].
Finally, research was started on two further aspects: the porosity of the SEI’s porous organic layer and the diffusion of the electrolyte's main solvent4 in this layer. The ongoing research should enable better estimation of the parameters used in phenomenological models, applied both in the automotive industry and at IFPEN for a better description of lithium battery aging.
1- Density functional theory is a method based on quantum physics that enables structures composed of several atoms to be studied and their physical-chemical properties including their chemical reactivity to be deduced.
2- Method used to simulate the behavior of systems evolving as a function of a master equation, using kinetic data related to elementary chemical reactions.
3- The GFN-xTB (Geometries, Frequencies, and Noncovalent interactions extended Tight-Binding) method is a semi-empirical quantum-mechanical approach for efficiently simulating molecular structures, reaction pathways and non-covalent interactions.
4- Ethylene carbonate.
References:
-
Mohammed Bin Jassar, Carine Michel, Sara Abada, Theodorus De Bruin, Sylvain Tant, Carlos Nieto-Draghi, Stephan N. Steinmann. A Joint DFT-kMC Study To Model Ethylene Carbonate Decomposition Reactions: SEI Formation, Growth, and Capacity Loss during Calendar Aging of Li-Metal Batteries, ACS Appl. Energy Mater., 6, (2023), 6934–45.
>> https://doi.org/10.1021/acsaem.3c00372
-
Maria Hellqvist Kjell, Sara Malmgren, Katarzyna Ciosek, Mårten Behm, Kristina Edström, Göran Lindbergh. Comparing aging of graphite/LiFePO4 cells at 22 °C and 55 °C – Electrochemical and photoelectron spectroscopy studies, J. Power Sources 243 (2013), 290–98.
>> https://doi.org/10.1016/j.jpowsour.2013.06.011
-
Bramy Pilipili Matadi, Sylvie Geniès, Arnaud Delaille, Thomas Waldmann, Michael Kasper, Margret Wohlfahrt-Mehrens, Frederic Aguesse, Emilie Bekaert, Isabel Jiménez-Gordon, Lise Daniel, Xavier Fleury, Michel Bardet, Jean-Frédéric Martin, Yann Bultel. Effects of Biphenyl Polymerization on Lithium Deposition in Commercial Graphite/NMC Lithium-Ion Pouch-Cells during Calendar Aging at High Temperature, J. Electrochem. Soc. 164, (2017): A1089.
>> https://doi.org/10.1149/2.0631706jes
-
Mohammed Bin Jassar, Carine Michel, Sara Abada, Theodorus De Bruin, Sylvain Tant, Carlos Nieto-Draghi, Stephan N. Steinmann. Lessons Learned from Semiempirical Methods for the Li-Ion Battery Solid Electrolyte Interphase, J. Phys. Chem. C, 128, (2024), 3269–80.
>> https://doi.org/10.1021/acs.jpcc.3c08176
Scientific contacts: theodorus.de-bruin@ifpen.fr and Carlos Nieto-Draghi
You may also be interested in
SC6 - How to better control loss of lithium battery capacity
Everybody knows that lithium-ion batteries, used in cell phones, computers, etc., gradually lose capacity and eventually fail. This loss of capacity is primarily due to a layer known as the SEI, which forms between one of the battery’s electrodes and the electrolyte (see Figure). This layer already appears after the first battery charge/discharge cycle, and grows over time, consuming lithium ions. The process is irreversible and therefore detrimental to battery capacitye...







