Thesis by Yuna Han: « Optimisation de la porosité et de l’acidité de zéolithes large pore pour la transformation de sucre en molécules plateforme » (Optimization of large-pore zeolite porosity and acidity for the conversion of sugars into platform molecules).
Biomass conversion into chemical products and intermediates is increasingly being adopted to reduce the carbon footprint of the industry concerned. Among biomass-based resources, sugars are extremely attractive since they contain a lot of functional groups enabling their conversion to products of interest (alcohols, acids, etc.). For example, through fructose dehydration, it is possible to obtain 5-hydroxymethylfurfural (5-HMF), a molecule that can be used to produce polymers. Since the conversion of fructose to 5-HMF is catalyzed by Brønsted acids1, liquid acid catalysts in solution are currently used, but without the possibility of recycling this catalyst. The development of a solid acid catalyst is thus crucially important.
Zeolites are porous materials that appear to be particularly interesting for this application, firstly because they are strong Brønsted acids, and secondly because their restricted pore space can limit the formation of undesirable oligo- or polymerized co-products. However, sugars are fairly bulky molecules and their diffusion in a zeolite’s micropores is slow, even for so-called “large-pore” zeolites such as faujasite. Accelerating this diffusion in the case of fructose would thus make it possible to increase catalytic activity for its conversion, and the production of a hierarchical porosity system, facilitating access to the micropores, is a way of achieving this. Such was the subject of this thesis.
In this thesis, treatments in basic media were used to generate a mesoporous porosity scale in USY-type (Ultra Stable Y zeolite) faujasite zeolites. Basic treatment selectively dissolves the silicon in the zeolite structure, without affecting the aluminum that generates the Brønsted acidity: this is known as desilication. It was necessary to find a compromise between mesopore generation and the destruction of the zeolite’s crystalline structure. These zeolites were then tested for the conversion of fructose into 5-HMF in order to evaluate the effect of the mesoporous system generated on catalyst activity and selectivity.
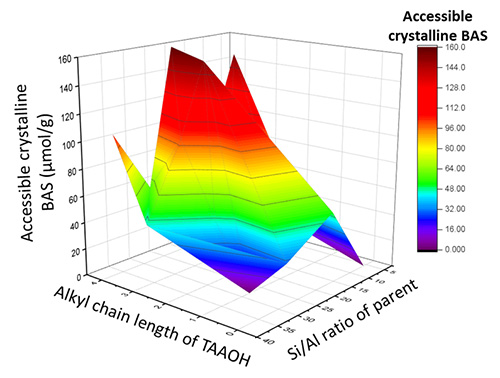
Initial work demonstrated how the addition of tetra-alkylammonium ions, with different alkyl chain lengths, during basic treatment, can regulate the degree of desilication and, therefore, mesopore formation. [1]. In this respect, the aluminum content of the parent zeolite also plays a very important role. (Figure 1).
The effect of mesopore generation on Brønsted acid site accessibility was then measured by adsorption of a basic molecule with a high steric hindrance, making it possible to define rules for the purposes of optimizing this property. While it intrinsically increases the rate of 5-HMF production from zeolite catalysts, the concentration of accessible acid sites does not, in itself, make it possible to explain the benefit of zeolites in fructose conversion. A chemometric analysis was carried out to look for other explanatory factors. In particular, the analysis identified low residual Lewis acidity2 and a low Al concentration in the catalyst, the latter possibly indicative of the hydrophobicity of the zeolite, as decisive criteria.
Another component of the research consisted in looking for fructose conversion reaction pathways. Analytical and spectroscopic techniques showed that 5-HMF is not a “primary” product, but that the reaction passes through dehydration intermediates of the cyclic form of fructose [2], providing a new insight into a debate hitherto unresolved in the literature.
This research has considerably advanced our knowledge of zeolite acid site porosity and accessibility modification. It has also provided a better understanding of the mechanisms involved in sugar conversion by zeolites. Nevertheless, some questions remain unanswered, such as a potentially important role of zeolite hydrophobicity in sugar conversion. This point has led to IFPEN initiating a new research program aimed at gaining a better understanding of the adsorption of water (a by-product of dehydration) in USYs with hierarchical porosity.
1- “Proton donor”-type acid.
2- A Lewis acid is a chemical species that can accept an electron pair and thus create a covalent bond.
References:
-
Han, Y. ; Larmier, K. ; Rivallan, M. ; Pirnruber, G., Generation of mesoporosity in H–Y zeolites by basic or acid/basic treatments: Towards a guideline of optimal Si/Al ratio and basic reagent, Microporous Mesoporous Mater., 2024, 365, 112906.
>> https://doi.org/10.1016/j.micromeso.2023.112906
-
[2] Han, Y.; Caillol, N.; Florent, A.; Mekki-Berrada, A.; Pirngruber, G.; Larmier, K., Dehydration of Fructose to 5-HMF Catalyzed by Commercial Faujasite Zeolites: Kinetic Study and Influence of the Properties of the Catalyst, ChemCatChem 2024.
>> https://doi.org/10.1002/cctc.202400257
Scientific contact: kim.larmier@ifpen.fr





