13.08.2024
3 minutes of reading
CCUS (Carbon Capture, Utilization and Storage) is a set of technologies that are crucial to the transition to a low-carbon economy. However, the infrastructure used to transport and store CO2, even in stainless steel, can corrode when exposed to this compound. To prevent these problems, a thorough understanding of the corrosion processes of the steels used is essential. CO2, which is transported in a supercritical state, is not normally aggressive to metallic materials, but the presence of water and other contaminants can lead to corrosion. The passive layer protecting stainless steel plays a crucial role in this process, as its properties determine the alloy's reactivity to the environment. A doctoral work has shed light on this complex issue.
In order to achieve a transition to a low-carbon economy, CCUS (Carbon Capture, Utilization and Sequestration) is emerging as a key technology, in particular capable of mitigating the environmental impact of heavy industry and coal-fired power plants. The infrastructures involved in CCUS, such as transport pipelines and storage tanks, are often built from stainless steel, because these materials have superior resistance to corrosion. However, despite the surface passivation layer1 that protects these alloys, they are not totally free from deterioration when exposed to severe conditions, particularly those very rich in CO2. Corrosion can then compromise the structural integrity of CCUS facilities, leading to leaks and failures that are both costly and potentially dangerous.
So, to prevent corrosion and ensure the durability and reliability of metal infrastructures, a thorough understanding of the corrosion mechanisms of stainless steels in these environments is crucial.
1 Mainly polycrystalline chromium oxide.
How CO2 interacts with the passive layer
To optimize the CO2transport, it is not carried as a gas, but in a supercritical state2, which is by itself not aggressive in its pure state to metallic materials. However, due to the presence of water and other contaminants, it may lead to corrosion of stainless steels [1]. To better understand and control this damaging phenomenon, it is important to know how CO2 interacts with the passive layer protecting the steel substrate. The properties (morphology and electronic structure) of this passive layer determine the surface chemical properties, and hence the reactivity of the alloy to the surrounding environment.
2 State of matter, obtained for CO2 by heating beyond its critical temperature (31.1 °C) and compression beyond its critical pressure (74 bar), characterized by an intermediate behavior between the liquid state and the gaseous state.
Molecular modeling to the rescue
In the framework of a PhD thesis3, the interactions between CO2 and the most abundant crystallographic orientations of the Cr2O3 oxide constituting the passivation layer were investigated using molecular modeling based on density functional theory4.
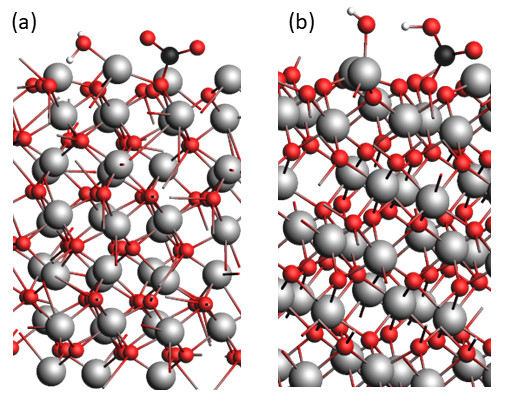
In particular, the simulations show that CO2 can adsorb favorably to the different surface orientations of Cr2O3 in the absence of water. This adsorption is accompanied by electron density transfer, leading to the formation of either a carbonate-type compound or a carboxylate [2]. Moreover, the presence of a pre-adsorbed water molecule on the surface thermodynamically favors CO2 adsorption (Figure 1a) [3]. However, the transfer of a proton from the water molecule to the CO2, forming a bicarbonate anion (HCO3-; Figure 1b), is highly unlikely as this reaction is kinetically unfavorable. The detachment and eventual dissolution of this anion would therefore not occur by this mechanism.
3 PhD thesis by A. Kumar, « Etude par modélisation moléculaire de la stabilité des couches passives des aciers », Université Paris Sciences et Lettres, 2021.
4 Density functional theory is a method based on quantum physics that enables structures composed of several atoms to be studied and their physico-chemical properties, including chemical reactivity, to be deduced
A passivation layer that still holds a few mysteries
An interesting complement to this work would be to consider a more representative state of the passivation layer of stainless steels, including the presence of crystalline defects in the surface chromium oxide. These are likely to modify the energy barriers, possibly favoring the formation of bicarbonates and their subsequent detachment, with a consequent decomposition of the protective passive layer.
References:
[1] F. Ropital, J. Kittel, “Corrosion Evaluation of Steels Under Geothermal Corrosion Evaluation of Steels under Geothermal CO2 Supercritical Conditions”. Proceedings World Geothermal Congress 2020, 2020.
[2] A. Kumar, F. Ropital, T. de Bruin, B. Diawara, “Effects of Surface Orientations of Cr2O3 on CO2 Adsorption”. Applied Surface Science 2020, 529, 147127.
>> DOI : https://doi.org/10.1016/j.apsusc.2020.147127
[3] A. Kumar, F. Ropital, T. de Bruin, B. Diawara, “Mechanistic Insights to CO2 Adsorption and Activation on Hydroxylated Chromia (0001) Surface”. Materials Today Communications 2024, 38 108224.
>> DOI : https://doi.org/10.1016/j.mtcomm.2024.108224
Scientific contacts : Theodorus.de-bruin@ifpen.fr ; Francois Ropital
You may also be interested in
Carbon steel in self-defense mode against corrosion
Carbon or low-alloy steel corrosion, by aqueous media containing CO2, hampers the development of numerous technologiesa f
Self-repair against localized corrosion
Due to their low cost and their good mechanical properties, carbon steels are a widely used material, including for equipments in contact with harsh environments, such as aqueous media containing CO2...
Self-repair against localized corrosion
Due to their low cost and their good mechanical properties, carbon steels are a widely used material, including for equipments in contact with harsh environments, such as aqueous media containing CO2...






