14.08.2024
3 minutes of reading
Ion transport in porous media occurs in natural phenomena, particularly in soils and rocks, and is also used in industry, to prepare catalysts for example, or decontaminate water. Since transport dynamics are heavily influenced by the interactions of ions with the surface of materials, a study of these interactions was carried out by researchers from IFPEN and LAGEPP, who have just published their results.
Ion transport in porous media is an important phenomenon to understand and measure:
- for numerous natural phenomena, such as the alteration of rock wettability or the retention of chemical species in soils;
- but also for industrial processes, such as active phase impregnation on catalyst supports or the use of adsorbents for water decontamination.
It is also known that ion interactions with the surface of a porous material strongly influence transport dynamics, i.e., the speed at which ions will penetrate and circulate within the material.
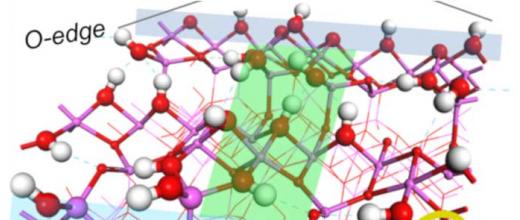
Nickel adsorption on an alumina grain: experimental set-up
A characterization of these ion-surface interactions was conducted by IFPEN, in partnership with the LAGEPP laboratory within the framework of PhD research . To achieve this, a porous granular solid (extruded cylindrical alumina catalyst support with a diameter of 1.44 mm) was brought into contact with nickel nitrate solutions in a perfectly stirred reactor. After equilibration of the surface with water, the nickel nitrate solution at fixed concentration and pH was circulated through the reactor, keeping the temperature, liquid flow rate and amount of solid present constant.
The pH, conductivity and nickel concentration of the solution exiting the reactor were monitored over time [1]. Samples of the solid at different residence times were taken to complete the description of the phenomena, providing both the nickel content within the solid and its spatial distribution at grain scale. This mapping of the nickel was obtained by LIBS (Laser Induced Breakdown Spectroscopy) on wet materials, a technique developed in the last few years by IFPEN in partnership with the Lyon Institute of Light and Matter [2].
1 Laboratoire d'Automatique, de Génie des Procédés et de Génie Pharmaceutique, Université de Lyon (Automation and Process and Pharmaceutical Engineering Laboratory, Lyon University).
2 Thesis by Rita Fayad, “Modélisation dynamique et étude expérimentale du transfert de matière d'ions métalliques et de leurs interactions avec les charges de surface à l'intérieur de structures poreuses de γ-alumine” (Dynamic modeling and experimental study of the matter transfer of metal ions and their interactions with surface charges inside porous γ-alumina structures), defended on 6 March 2024 at Lyon 1 University
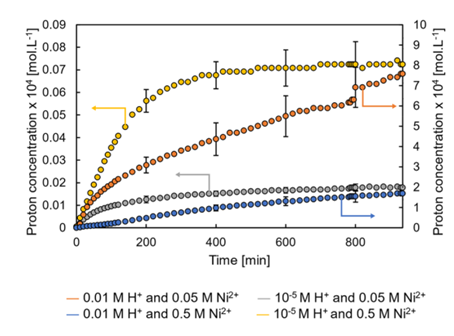
Evolution of pH solution exiting the reactor
Figure 1 shows the monitoring over time of pH (proton concentration) at the reactor outlet for two nickel molar concentrations (0.05 and 0.5 mol/L) and two different acidity levels (pH2 and pH5) at the inlet. When exiting the reactor, the proton concentration is very low to begin with (around 10-7 mol/L). It then increases and stabilizes with very slow dynamics, particularly when the initial pH of the solution is low (blue and orange curves on figure 1). This is due to the fact that protons, which are highly concentrated entering the reactor, diffuse into the core of the alumina support and gradually interact with its surface, until it is eventually saturated.
The story of an alumina grain illustrated in images
The LIBS analysis of alumina extrudates is illustrated in figure 2. For low nickel concentrations, competition between H+ protons and Ni2+ nickel ions to interact with the alumina surface leads, over a long period of time, to “egg-yolk” profiles, with the nickel located at the periphery of the grains gradually being driven out of the solid by the protons.
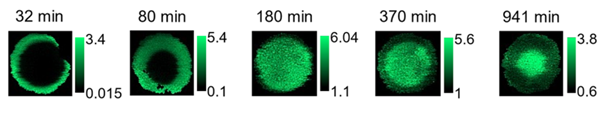
The slowness of the dynamics at low pH levels was explained by the alumina surface charge: highly positive in this case, it repels the protons, themselves positively charged..
A new model to incorporate the phenomena observed
The wealth of information obtained both for the liquid phase and the solid was used to develop a model taking into account these surface charge and adsorption competition phenomena, explaining the experimental results obtained. Publication of this model is pending.
References:
[1] R.Fayad, F. Couenne, L. Sorbier, E. Jolimaitre, C.-P. Lienemann, A. Galfre, C. Jallut, M. Tayakout-Fayolle, "Adsorption of Nickel Ions on the γ-Alumina Surface: Competitive Effect of Protons and Its Impact on Concentration Profiles", Langmuir 40 3343–3353 (2024)
>> DOI : https://doi.org/10.1021/acs.langmuir.3c02199.
[2] L. Jolivet, L. Catita, O. Delpoux, C.-P. Lienemann, L. Sorbier, V. Motto-Ros , J. Catal. "Direct multi-elemental imaging of freshly impregnated catalyst by Laser-Induced Breakdown Spectroscopy" 401 183–187 (2021)
>> DOI : https://doi.org/10.1016/j.jcat.2021.07.010
Scientific contact: Loic Sorbier
You may also be interested in
Spectroscopy and quantum calculations uncover the secrets of alumina supports
SC3 - Simulation of the adsorption/transport combination via a generalized lattice-boltzmann approach
Transport of molecules within porous structures that adsorb on the surfaces plays an important role in numerous contexts and very different applications. These include pollutant transport in soils, industrial catalytic conversion and purification processes, and chromatographic techniques...
A la recherche d’un nouveau chemin réactionnel pour l’oligomérisation de l’éthylène par le nickel
La réaction d’oligomérisation des oléfines permet d’accéder à une large gamme de composés clés dans le domaine des carburants, de la pétrochimie ou encore de la chimie fine...